Most days we read CMS regulation and we are not that surprised by what they print. We were given plenty of heads up related to PDPM. CMS has been mostly forthright about the COVID waivers. Generally speaking, the RAI User’s Manual is a consistent guide to completing the MDS. The State operations manual and survey critical element pathways are straight forward guide to survey agencies as they conduct surveys. We have come to rely on a usually consistent message so that the data is mostly consistent as well.
However, with the recent memo issuance and subsequent implementation of the CMS vaccine mandates plus the addition of nursing home weekend staffing and turnover data publicly posted on Care Compare in January 2022 with a revised 5-star user’s guide, we noticed somewhat inconsistent messaging from CMS so we began to take a closer look at a known issue. The issue is significant inconsistencies between CMS regions and states when it comes to the state cut points for health inspection star ratings. Simply put, these ongoing inconsistencies have driven us to ask, “why?”.
In a recent memo, QSO-22-12-ALL, issued directly on the heels of the vaccine mandate memos noted above, CMS specifically addresses, “State Obligations to Survey to the Entirety of Medicare and Medicaid Health and Safety Requirements under the 1864 Agreement.” The Memo summary notes the following three key points.
- ”The 1864 Agreement is the agreement between CMS and the State survey agency to carry out the provisions of Sections 1864, 1874, and related provisions of the Social Security Act (Act). This 1864 Agreement specifies the functions to be performed by the State. The requirements for survey and certification procedures are provided in 42CFR Part 488.”
- ”Specifically, under Article II, A.1.(c), the State duties include “surveying for the purpose of certifying to the Secretary the compliance or non-compliance of providers and suppliers of services and resurveying such entities, at such times and manner as the Secretary may direct.”
- ”CMS, as delegated by the Secretary, designates the content of the survey process to be followed by States.”
In other words, in this memo, CMS is appealing to state survey agencies for consistency in the application of survey guidance found in the state operations manual, critical element pathways etc. The tenor of this memo issued so close on the heels of the vaccine mandate guidance for new survey tag F888 (§483.80 Infection control, §483.80(i) COVID-19 Vaccination of facility staff. The facility must develop and implement policies and procedures to ensure that all staff are fully vaccinated for COVID-19), seems telling. In particular, CMS seems to be requiring consistency with surveying the vaccine mandate TAG F888 but also, by proxy, the rest of the SOM survey guidance.
This is where we began to ask fresh questions surrounding the known health inspection cut point issue for the following reasons. First CMS indicates in QSO-22-12-ALL the following related to consistency among the state survey agencies,
States that fail to perform survey and certification functions in a manner sufficient to assure the CMS of the full certification of compliance with all Conditions of Participation, Conditions for Coverage, and Requirements for Participation for providers and suppliers, may, among other things, receive a revised Survey and Certification budgetary allocation. The Medicare and Medicaid certification of providers and suppliers in a State whose oversight process is substantially deficient may be jeopardized if CMS cannot ensure that the regulatory minimum health and safety standards have been met. Also, CMS will provide additional information to providers in such states clarifying the expected process to demonstrate compliance with federal requirements.”
Centers for Medicare & Medicaid ( QSO-22-12-ALL )
It almost appears that CMS knows there is an issue with inconsistent with interpretation and application of survey guidance in the various states. CMS goes on in this memo to state,
In making the Survey and Certification budgetary allocation, CMS may, among other things, adjust the amount allocated to States that refuse to survey and certify compliance with all applicable Medicare and Medicaid health and safety regulations.”
Centers for Medicare & Medicaid ( QSO-22-12-ALL )
CMS is serious. But why the inconsistency in the cut points for each state?
Enter the recently revised 5-Star user’s guide. CMS indicates the following related to the known inconsistency in state health inspection cut points reflective of state survey agencies. This is not new language.
Health inspections are based on federal regulations, which surveyors implement using national interpretive guidance and a federally specified survey process. Federal staff train state inspectors and oversee state performance. The federal oversight includes quality checks based on a 5 percent sample of the health inspections performed by states, in which federal inspectors either accompany state inspectors or replicate the inspection within 60 days of the state and then compare results. These control systems are designed to improve consistency in the survey process. Nonetheless there remains variation among states in both inspection process and outcomes. Such variation derives from many factors, including:
- Survey Management: Variation among states in the skill sets of inspectors, supervision of inspectors, and the inspection processes;
- State Licensure: State licensing laws set forth different expectations for nursing homes and affect the interaction between state enforcement and federal enforcement (for example, a few states conduct many complaint investigations based on state licensure, and issue citations based on state licensure rather than on the federal regulations);
- Medicaid Policy: Medicaid pays for the largest proportion of long-term care in nursing homes. Nursing home eligibility rules, payment, and other policies in the state-administered Medicaid program may be associated with differences in survey outcomes
In other words, surprisingly, CMS knows there will be inconsistency in the state cut points and is allowing it based of the above stated reasons. How can this be in light of the recent directives in QSO-22-12-ALL related to survey standardization? In the recent memo it appears that state survey agencies will be held to a strict standard of survey compliance relative to the SOM while in the 5-Star user’s guide CMS allows that there will be inconsistencies. We grant that there will be some differences between states. No two nursing homes are alike, and no two survey teams always apply the SOM interpretive guidance exactly the same.
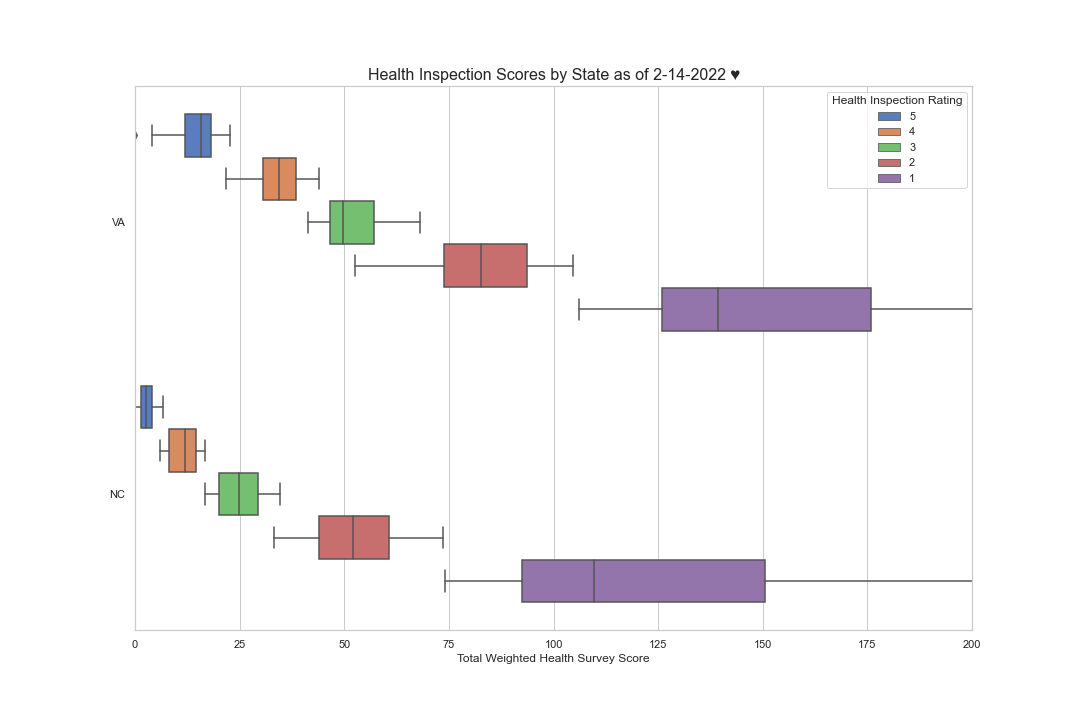
However, The significant differences in health inspection cut point levels from CMS region to CMS region and more tellingly, from state to state, seem to tell a vastly different story. For example, did you know that nursing homes in Tennessee must achieve a composite score of 4.667 or less over the most recent 3 survey cycles to achieve a 5-Star health inspection rating, while in Virginia, a nursing facility only has to achieve a composite score of 20.667. That’s a 23% differential in favor of Virginia. Here are a few more examples. For a 5-star health inspection rating nursing homes in North Carolina can only score 6 points to achieve a 5-star health inspection rating while Washington state only nursing homes only have to achieve 44.667 points, in Michigan, 34.667 and in New Hampshire, you can only score 2.667 points to achieve a 5-star health inspection rating. That’s pretty tough.
Here is an interesting look at North Carolina and Virginia. Note that in North Carolina most of the 4 star buildings are in the 5-star range and the 3-star facilities are in the 4 star range in Virginia. How can this be?
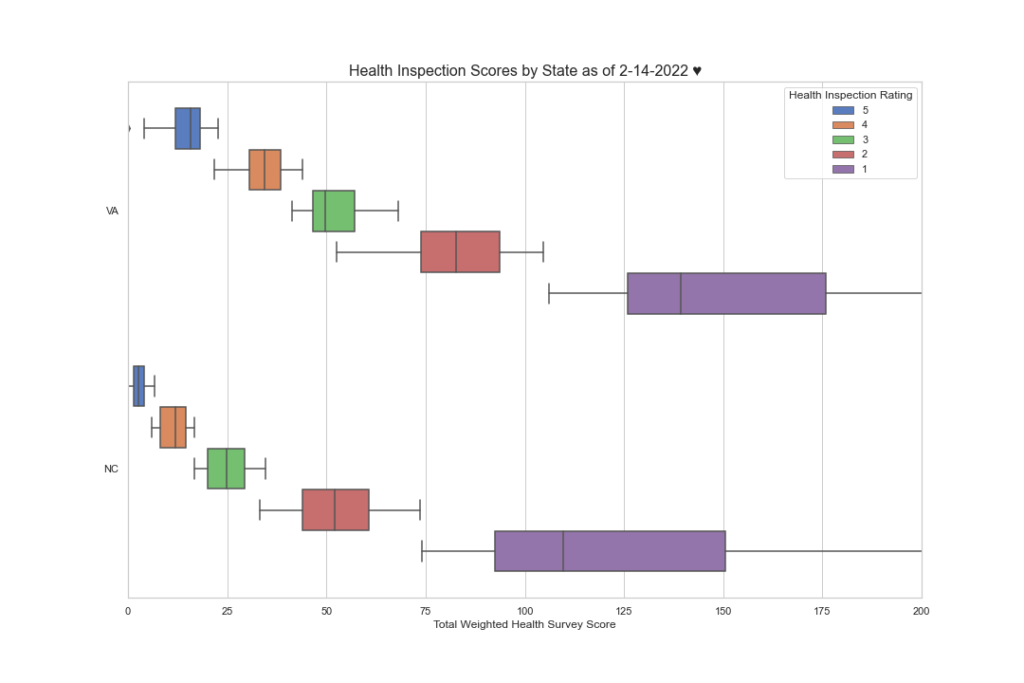
Here is the same data but by CMS region. Note the disparities here as well.
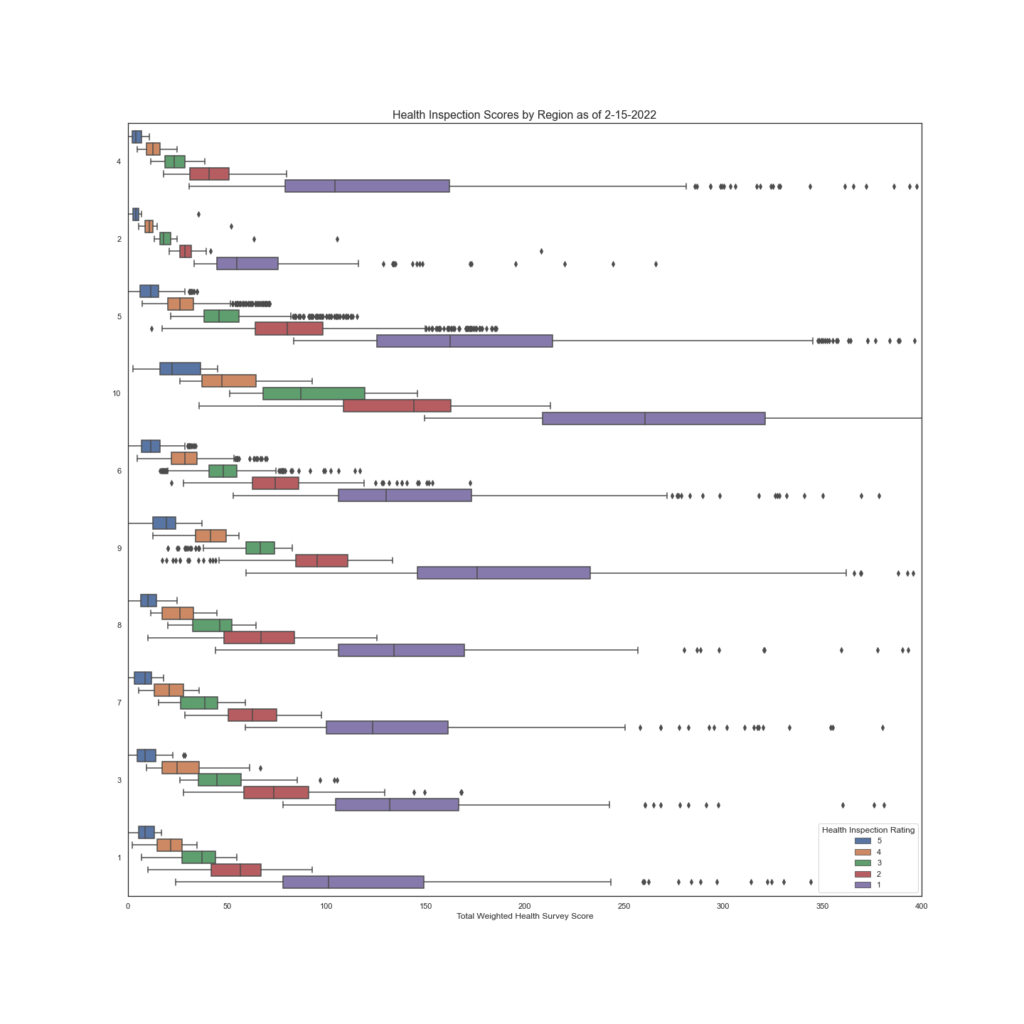
While it appears that there may be some consistency between a few regions, note regions 7, 1 and 8, a deeper look into the individual states tells a different story.
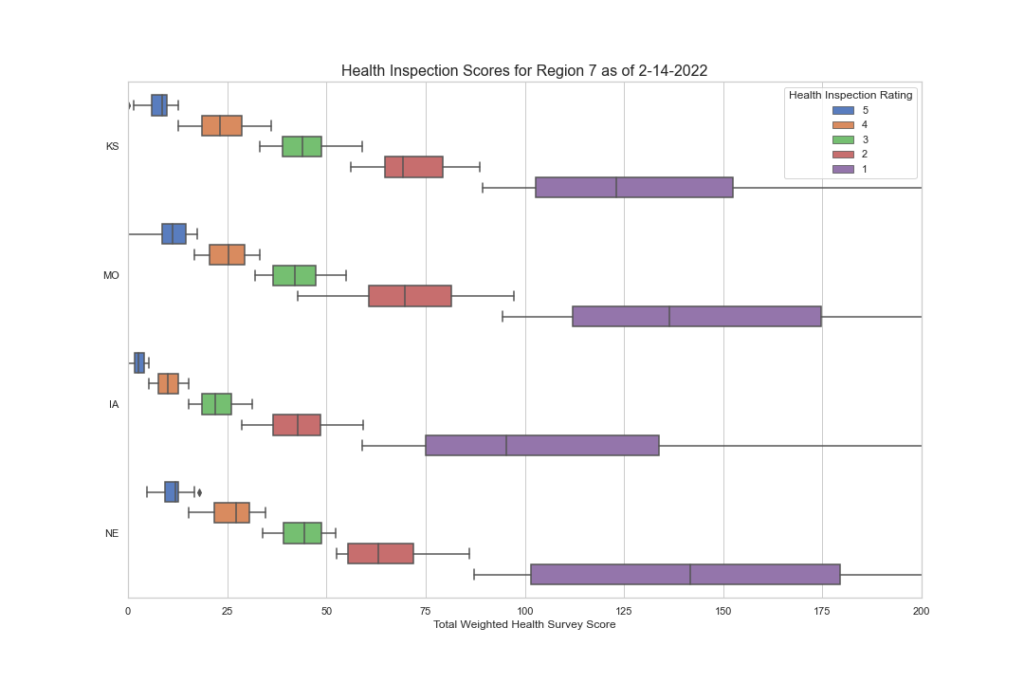
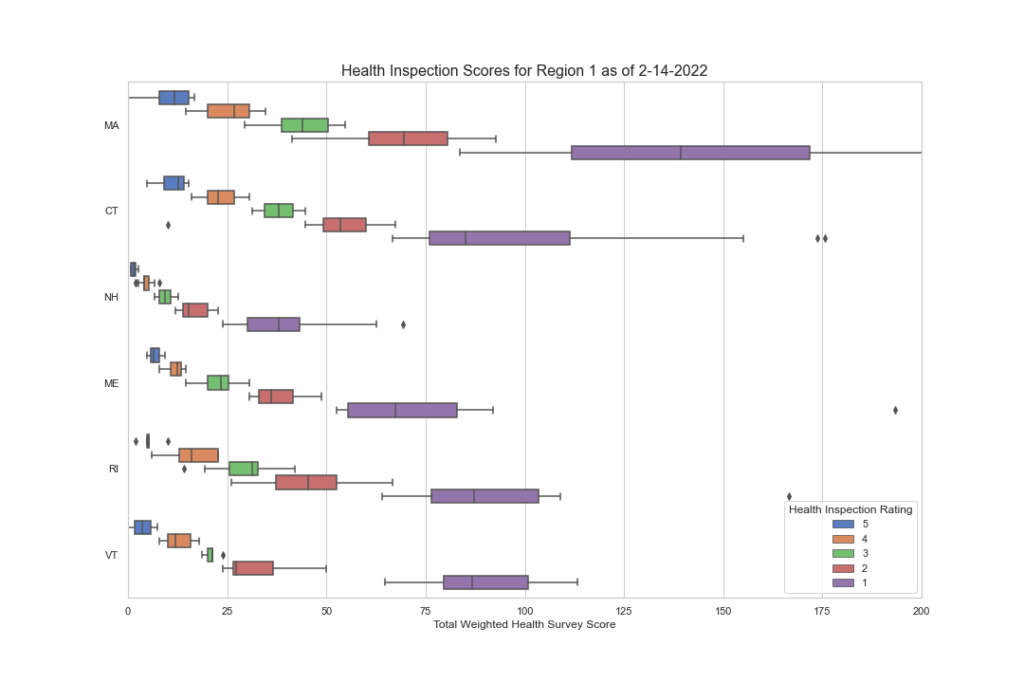
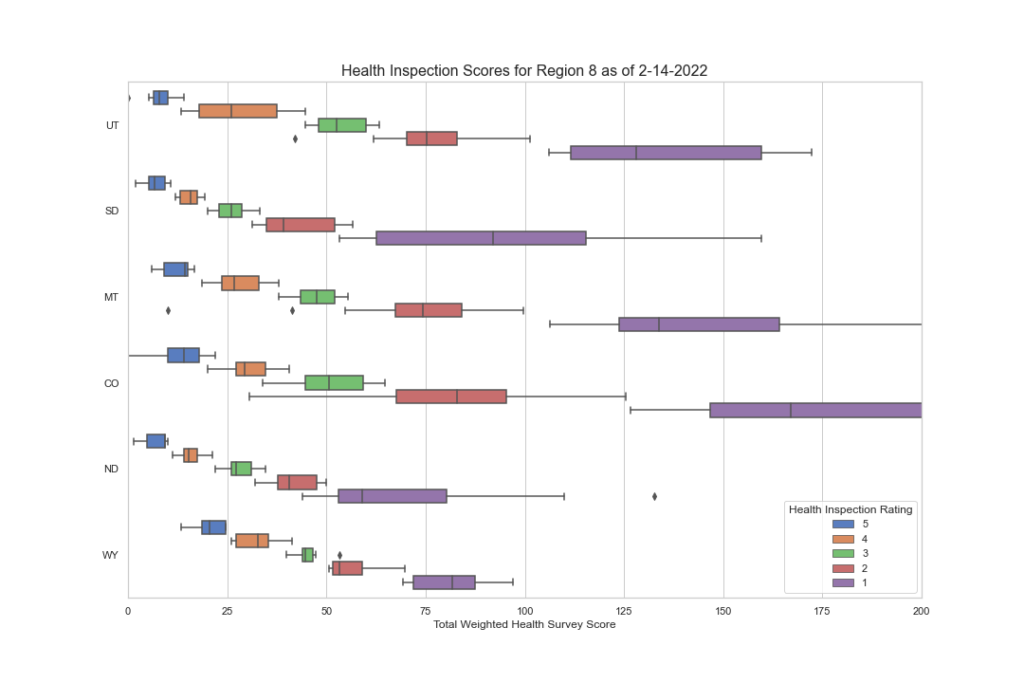
I hope you’re finding this data as interesting as we do. But there’s more to discover as we dig deeper. We want to know what F tags are cited most often in each region and state? Are they vastly different? Are there wide disparities among states and regions in the scope and severity? We have begun to peak into this, and the initial results are worth looking at. In the next blog segment, we will uncover this data more and try to draw some conclusions as to what the data is saying. If CMS wants consistency, this data demands a second look.